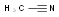
-
乙腈
NMR and HPLC COA下载 MSDS下载 - Names:
Acetonitrile
- CAS号:
75-05-8
MDL Number: MFCD00001878 - MF(分子式): C2H3N MW(分子量): 41.05
- EINECS:200-835-2 Reaxys Number:
- Pubchem ID:6342 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| JY0313-500ml | 500ml | AR | ¥ 58.00 | ¥ 58.00 | Instock | ¥ 0.00 |
| 中文别名 | 乙腈(75-05-8,Acetonitrile);氰甲烷;乙烷腈;色谱乙腈;甲基氰 |
| 英文别名 | Acetonitrile(75-05-8);Methyl cyanide;Cyanomethane;Ethanenitrile |
| CAS号 | 75-05-8 |
| Inchi | InChI=1S/C2H3N/c1-2-3/h1H3 |
| InchiKey | WEVYAHXRMPXWCK-UHFFFAOYSA-N |
| 分子式 Formula | C2H3N |
| 分子量 Molecular Weight | 41.05 |
| 溶解度Solubility | 与水、甲醇、四氯化碳、乙酸甲酯、乙酸乙酯、二氯乙烷及许多非饱和烃类溶剂互溶 |
| 性状 | 无色透明液体 |
| 储藏条件 Storage conditions | 密封阴凉处保存。 |
乙腈(75-05-8,Acetonitrile)毒性测试:
| 生物 | 测试类型 | 路线 | 剂量 | 影响 | 参考 |
| women | TDLo | oral | 500 mg/kg (500 mg/kg) | BEHAVIORAL: COMA; CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Postgraduate Medical Journal., 73(299), 1997 [PMID:9196706] |
| child | TDLo | oral | 800 mg/kg (800 mg/kg) | BEHAVIORAL: HALLUCINATIONS, DISTORTED PERCEPTIONS; BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: NAUSEA OR VOMITING | American Journal of Emergency Medicine., 9(268), 1991 [PMID:2018601] |
| man | TDLo | oral | 571 mg/kg (571 mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD; GASTROINTESTINAL: NAUSEA OR VOMITING | Acta Pharmacologica et Toxicologica, Supplementun., 41(340), 1977 |
| man | TDLo | oral | 64 mg/kg (64 mg/kg) | BEHAVIORAL: EXCITEMENT | Journal of Toxicology, Clinical Toxicology., 29(447), 1991 [PMID:1749050] |
| human | TCLo | inhalation | 160 ppm/4H (160 mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Toxicology of Drugs and Chemicals, Deichmann, W.B., New York, Academic Press, Inc., 1969, -(65), 1969 |
乙腈(75-05-8,Acetonitrile)实验注意事项:
1.使用303-98-0实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.使用303-98-0实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害。
3.取样品303-98-0的移液枪头需及时更换,必要时为避免交叉污染尽可能选择滤芯吸头。
4.称量药品时选用称量纸,并无风处取药和称量以免扬撒,试剂的容器使用前务必确保干净,并消毒。
5.取药品303-98-0时尽量采用多个药勺分别使用,使用后清洗干净。
6.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染。
大规格定制:定制产品请将信息发送至sales@bio-fount.com。
Acetonitrile(75-05-8) Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:乙腈(75-05-8,Acetonitrile),乙腈试剂,乙腈中间体,乙腈的作用,乙腈的用途,乙腈的外观,乙腈的溶解度,乙腈溶剂,乙腈的合成,乙腈的生产,乙腈的MSDS,乙腈的制造,乙腈的注意事项
| 产品说明 | 乙腈(75-05-8)具有极性非质子传递溶剂和EC 3.5.1.4(酰胺酶)抑制剂的作用.乙腈可用于制造胰岛素,抗生素和维生素,乙腈溶解度,乙腈msds,乙腈结构式详见主页. |
| Introduction | Acetonitrile (75-05-8,乙腈) is prepared by heating a mixture of acetamide and glacial acetic acid. It is an important industrial solvent and is mainly used as a medium for organic synthesis. |
| Application1 | 乙腈(75-05-8,Acetonitrile)可用于制造香水。 |
| Application2 | 乙腈(75-05-8,Acetonitrile)用作溶剂,并用于制造其他化学品,纤维,塑料和染色织物。 |
| Application3 | 乙腈(75-05-8,Acetonitrile)可用于制造胰岛素,抗生素和维生素等。 |
| 警示图 | |
| 危险性 | Danger |
| 危险性警示 | |
| 安全声明 | H225,H302,H312,H319,H332 |
| 安全防护 | P210,P280,P305+P351+P338 |
| 备注 |
| 象形图 |   |
|---|---|
| 信号警告 | Danger |
| GHS危险说明 |
H225: Highly Flammable liquid and vapor [Danger Flammable liquids] H302: Harmful if swallowed [Warning Acute toxicity, oral] H312: Harmful in contact with skin [Warning Acute toxicity, dermal] H319: Causes serious eye irritation [Warning Serious eye damage/eye irritation] H332: Harmful if inhaled [Warning Acute toxicity, inhalation] |
| 防范说明代码 |
P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P280, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P312, P322, P330, P337+P313, P363, P370+P378, P403+P235, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| C13, Compounds with C-O double bounds(C=O)(Chemical Shifts for Oxygen-17,2002) |
| Acetonitrile Adduct Formation as a Sensitive Means for Simple Alcohol Detection by LC-MS(Journal of The American Society for Mass Spectrometry,2014) |
| C8-C100, Nitrogen-oxygen compounds(Chemical Shifts for Oxygen-17,2002) |
| Study of complexation process between N-phenylaza-15-crown-5 with yttrium cation in binary mixed solvents(Journal of Inclusion Phenomena and Macrocyclic Chemistry,2011) |
| Calculation of Vibrational Spectra for Coordinated Thiocyanate Ion in Acetonitrile(Journal of Applied Spectroscopy,2016) |
Adsorption of Acetonitrile on Platinum and its Effects on Oxygen Reduction Reaction in Acidic Aqueous Solutions—Combined Theoretical and Experimental Study
Abstract:Combined theoretical and experimental study of acetonitrile (AcN) adsorption on platinum was performed and its effects on the kinetics of oxygen reduction reaction in HClO4 and H2SO4 solutions were examined. Using periodic density functional theory calculations, it was shown that AcN molecule can interact with Pt surface either through the unsaturated π electron system or via lone electron pair of nitrogen atom. In both cases, adsorption energy decreases upon increasing coverage, while the modification of electronic structure of Pt surface is localized to the adsorption site. By combining the results of the DFT calculations with the results of blank cyclic voltammetry and rotating disk electrode voltammetry in O2-saturated solutions, it was concluded that the effects of AcN on Pt surface chemistry and ORR kinetics are primarily steric in nature. Resulting measured ORR activities of polycrystalline platinum in the presence of AcN are due to the combination of (i) suppression of (bi)sulfate adsorption (in H2SO4 solution), (ii) suppression of surface oxidation (in both H2SO4 an HClO4 solution), and (iii) site blockage by adsorbed AcN (or products of its electrochemical transformations).
Calculation of Vibrational Spectra for Coordinated Thiocyanate Ion in Acetonitrile
Abstract:The impact of the association of lithium cation with NCS– ion in acetonitrile on the vibrational spectrum was studied by the density-functional method in the B3LYP/6-31+G(d,p) approximation. The best agreement between experimental and calculated ionic association data was achieved taking into account the nonspecific solvation, oversolvation, and solubility of ionic complexes within the discrete-continuum model. The microstructures of the thiocyanate ion in a contact ion pair with lithium cation and ion-pair dimer and trimer in acetonitrile were established.
Study of complexation process between N-phenylaza-15-crown-5 with yttrium cation in binary mixed solvents
Abstract:The complexation reaction between Y3+ cation with N-phenylaza-15-crown-5(Ph-N15C5) was studied at different temperatures in acetonitrile–methanol (AN/MeOH), acetonitrile–propanol (AN/PrOH), acetonitrile–1,2 dichloroethane (AN/DCE) and acetonitrile–water (AN/H2O) binary mixtures using the conductometric method. The results show that in all cases, the stoichiometry of the complex is 1:1 (ML). The values of formation constant of the complex which were determined using conductometric data, show that the stability of (Ph-N15C5.Y)3+ complex in pure solvents at 25 °C changes in the following order: PrOH > AN > MeOH and in the case of binary mixed solutions at 25 °C it follows the order: AN–DCE > AN–PrOH > AN–MeOH > AN–H2O. The values of standard thermodynamic quantities (?H °c and ?S °c) for formation of (Ph-N15C5.Y)3+ complex were obtained from temperature dependence of the formation constant using the van’t Hoff plots. The results show that in most cases, the complex is entropy and enthalpy stabilized and these parameters are influenced by the nature and composition of the mixed solvents. In most cases, a non-linear behavior was observed for variation of log Kf of the complex versus the composition of the binary mixed solvents. In all cases, an enthalpy–entropy compensation effect was observed for formation of (Ph-N15C5.Y)3+ complex in the binary mixed solvents.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



