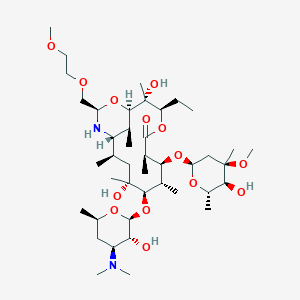
-
地红霉素
- names:
Dirithromycin
- CAS号:
62013-04-1
MDL Number: MFCD00865041 - MF(分子式): C42H78N2O14 MW(分子量): 835.07
- EINECS:624-080-7 Reaxys Number:
- Pubchem ID:6473883 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000154-500mg | 500mg | >98.0% | ¥ 633.75 | ¥ 633.75 | 2-3天 | ¥ 0.00 |
| 中文别名 | 地红霉素(62013-04-1);地霉素;LY-237216;LY 237216;LY237216;地红霉素; 抗生素AS-E 136;(9S)-9-脱氧-11-脱氧-9,11-(亚氨基((1R)-2-(2-甲氧基乙氧基)亚乙基)氧基)红霉素; |
| 英文别名 | Dirithromycin(62013-04-1);LY-237216; LY 237216; LY237216; Dirithromycin; Antibiotic AS-E 136;(9S)-9-deoxo-11-deoxy-9,11-(imino((1R)-2-(2-methoxyethoxy)ethylidene)oxy)erythromycin;dirithromycin;Dynabac;LY 237216;LY-237216;Nortron; |
| CAS号 | 62013-04-1 |
| Inchi | InChI=1S/C42H78N2O14/c1-15-29-42(10,49)37-24(4)32(43-30(56-37)21-52-17-16-50-13)22(2)19-40(8,48)36(58-39-33(45)28(44(11)12)18-23(3)53-39)25(5)34(26(6)38(47)55-29)57-31-20-41(9,51-14)35(46)27(7)54-31/h22-37,39,43,45-46,48-49H,15-21H2,1-14H3/t22-,23-,24+,25+,26-,27+,28+,29-,30-,31+,32+,33-,34+,35+,36-,37?,39+,40-,41-,42-/m1/s1 |
| InchiKey | WLOHNSSYAXHWNR-GETPLZSYSA-N |
| 分子式 Formula | C42H78N2O14 |
| 分子量 Molecular Weight | 835.07 |
| 溶解度Solubility | 生物体外In Vitro:Ethanol : ≥ 50 mg/mL(59.88 mM)DMSO溶解度33.33 mg/mL(39.91 mM;Need ultrasonic)*"≥" means soluble可溶, but saturation unknown溶解度未知. |
| 性状 | 白色固体粉末,Power |
| 储藏条件 Storage conditions | -20°C,3 years年 4°C 2 years年 / In solvent溶液中:-80°C 6 months月 -20°C 1 month月 |
地红霉素(62013-04-1,Dirithromycin,LY 237216)毒性摘要:
The toxic symptoms following an overdose of a macrolide antibiotic may include nausea, vomiting, epigastric distress, and diarrhea.
地红霉素(62013-04-1,Dirithromycin,LY 237216)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:地红霉素试剂,地红霉素合成,地红霉素杂质,地红霉素中间体,地红霉素闪点,地红霉素熔点,地红霉素密度,地红霉素溶解度,地红霉素旋光度,地红霉素购买,

| 产品说明 | 地红霉素(62013-04-1,Dirithromycin,LY 237216)是一种大环内酯类糖肽抗生素,通过与70S细菌核糖体的50S亚基结合来抑制肽的转运. |
| Introduction | 地红霉素(62013-04-1,Dirithromycin,LY 237216)is a macrolide glycopeptide antibiotic by binding to the 50S subunit of the 70S bacterial ribosome to inhibit the translocation of peptides. |
| Application1 | Dirithromycin与70S细菌核糖体的50S亚基结合,从而抑制了肽的转运。 |
| Application2 | |
| Application3 |
1、地红霉素(LY 237216)是一种大环内酯糖肽类抗生素,通过与70S细菌核糖体的50S亚基结合来抑制肽的转运。靶标:抗菌霉素是一种新的大环内酯,其体外抗菌活性的光谱和程度类似于红霉素。与红霉素相比,地红霉素具有更长的消除半衰期,可以每天给药一次,并且在某些组织中还可以实现更高的细胞:细胞外浓度比和更高的浓度。多中心双盲临床试验显示,在治疗呼吸道以及皮肤和软组织的简单细菌感染中,地霉素与红霉素的疗效相似。地霉素具有一些诱人的药代动力学特性。与红霉素相比,地霉素的消除半衰期长,因此可以每天给药一次,并且组织浓度更高,更长。地红霉素的活性谱,不良反应,临床疗效和细菌清除率可能与红霉素相似。
2、地红霉素是一种大环内酯类糖肽抗生素,通过与70S细菌核糖体的50S亚基结合来抑制肽的转运.Dirithromycin与70S细菌核糖体的50S亚基结合,从而抑制了肽的转运。
3、地红霉素是一种半合成的大环内酯类抗生素前药。地霉素在肠道吸收过程中通过水解转化为具有微生物活性的红霉素胺。乙丙胺与易感生物体70 S核糖体的50 S亚基结合,从而抑制细菌RNA依赖性蛋白的合成。这种抗生素用于治疗由革兰氏阳性微生物(包括金黄色葡萄球菌,肺炎链球菌和化脓性细菌),革兰氏阴性微生物(包括流感嗜血杆菌,嗜肺乳杆菌)引起的呼吸道,皮肤和软组织感染。卡他氏菌和肺炎支原体。
4、 地红霉素是由红霉素衍生物(9S)- 红霉素环胺与2-(2-甲氧基乙氧基)乙醛缩合产生的半缩醛。由于含半氨基的恶嗪环在酸性和碱性条件下均不稳定,因此,红霉素对(9S)-红霉素具有更高的脂溶性前药作用。以肠溶片的形式给药,可保护其免受酸在胃中的水解,可用于治疗易感生物引起的呼吸道,皮肤和软组织感染。它具有前药作用。
5、地红霉素是一种大环内酯类糖肽抗生素,用于治疗许多不同类型的细菌感染,例如支气管炎,肺炎,扁桃体炎,甚至皮肤感染。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303吞入可能有害+H313皮肤接触可能有害+H2413吸入可能对身体有害 |
| 安全防护 | P264处理后彻底清洗+P280戴防护手套/穿防护服/戴防护眼罩/戴防护面具+P305如果进入眼睛+P351用水小心冲洗几分钟+P338取出隐形眼镜(如果有)并且易于操作,继续冲洗+P337如果眼睛刺激持续+P2393获得医疗建议/护理 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| 象形图 |  |
| 信号 | Warning |
| GHS危险说明 | Aggregated GHS information provided by 38 companies from 1 notifications to the ECHA C&L Inventory. |
| H317 (100%): May cause an allergic skin reaction [Warning Sensitization, Skin] | |
| Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown. | |
| 防范说明代码 | P261, P272, P280, P302+P352, P321, P333+P313, P363, and P501 |
| (The corresponding statement to each P-code can be found at the GHS Classification page.) |
| Brogden, R.N. and D.H. Peters, Dirithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic efficacy. Drugs, 1994. 48(4): p. 599-616. |
| Wintermeyer, S.M., S.M. Abdel-Rahman, and M.C. Nahata, Dirithromycin: a new macrolide. Ann Pharmacother, 1996. 30(10): p. 1141-9. |
| Sides, G.D., et al., Pharmacokinetics of dirithromycin. J Antimicrob Chemother, 1993. 31 Suppl C: p. 65-75. |
| Investigation of the in-vitro uptake, intraphagocytic biological activity and effects on neutrophil superoxide generation of dirithromycin compared with erythromycin PMID 1337069; The Journal of antim |
| The new macrolide antibiotics: azithromycin, clarithromycin, dirithromycin, and roxithromycin PMID 1318761; The Annals of pharmacotherapy 1992 Jan; 26(1):46-55 (Review Article) Name matches: azithromy |
1.Quantitative determination of erythromycylamine in human plasma by liquid chromatography-mass spectrometry and its application in a bioequivalence study of dirithromycin.
Liu YQ1, Chen QY, Chen BM, Liu SG, Deng FL, Zhou P. J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Mar 15;864(1-2):1-8. doi: 10.1016/j.jchromb.2007.12.021. Epub 2008 Jan 4.
A sensitive, rapid liquid chromatographic-electrospray ionization mass spectrometric method for determination of erythromycylamine in human plasma was developed and validated. Erythromycylamine in plasma (0.2 mL) was extracted with ethyl acetate, the organic phase was transferred to another clear 1.5 mL Eppendorf tube and evaporated to dryness under gentle nitrogen stream at 45 degrees C, and the residue was dissolved in 100 microL of mobile phase. The samples were separated using a Thermo Hypersil HyPURITY C18 reversed-phase column (150 mm x 2.1 mm I.D., 5 microm). A mobile phase containing 10 mM of ammonium acetate (pH = 6.4)-acetonitrile-methanol (50:10:40, v/v/v) was used isocratically eluting at a flow rate of 0.2 mL/min. Erythromycylamine and its internal standard (IS), midecamycin, were measured by electrospray ion source in positive selective ion monitoring mode. The method demonstrated that good linearity ranged from 4.5 to 720 ng/mL with r = 0.
2.Long-acting erythromycins: assessing their role in treating outpatient odontogenic infections.
Alexander RE1, Grogan DM. Tex Dent J. 2009 Apr;126(4):326-33.
Erythromycins have been part of our armamentarium against selected bacterial infections since they were discovered in 1952 and approved by the Food and Drug Administration (FDA) in 1964. In 1991, two newer, long-acting erythromycin analogues, azythromycin (brand name: Zithromax) and clarithromycin (brand name: Biaxin) were approved by the FDA. They were joined a few years later by a third long-acting form, dirithromycin (brand name: Dynabac).
3.Structural Correspondence of the Oriented Attachment Growth Mechanism of Crystals of the Pharmaceutical Dirithromycin.
Liang Z1, Wang Y1, Wang W1, Han X1, Chen JF1, Xue C1, Zhao H1. Langmuir. 2015 Dec 29;31(51):13802-12. doi: 10.1021/acs.langmuir.5b02901. Epub 2015 Dec 15.
The oriented attachment (OA) mechanism is promising for designing novel nanomaterials, yet an intensive understanding of the relationship between the crystal structure and attachment orientation is still lacking. In this work, we report layered hexagonal crystals of the pharmaceutical dirithromycin (DIR) containing multiple layers fabricated via a solvothermal method for a certain period of time at 40 °C. These elongated hexagonal crystals experience an OA that is preferentially on the face (001) of the initial crystals to assemble the final crystals into layered stacks. Through agreement with molecular modeling calculations, we predicted the final crystal growth morphology and confirmed the favored attachment surface based on the energy change ΔE following an OA event. These simulation results at the molecular level yielded good agreement with the crystal growth experiments. This study demonstrates the critical importance of combining experiments with a computational approach to understand the intrinsic molecular details of the OA growth mechanism of other compounds and to design nanomaterials with a desirable morphology and physical and chemical properties.
4.Epigallocatechin gallate as a modulator of Campylobacter resistance to macrolide antibiotics.
Kurin?i? M1, Klan?nik A, Smole Mo?ina S. Int J Antimicrob Agents. 2012 Nov;40(5):467-71. doi: 10.1016/j.ijantimicag.2012.07.015. Epub 2012 Sep 20.
Comprehensive therapeutic use of macrolides in humans and animals is important in the selection of macrolide-resistant Campylobacter isolates. This study shows high co-resistance to erythromycin, azithromycin, clarithromycin, dirithromycin and tylosin, with contributions from the 23S rRNA gene and drug efflux systems. The CmeABC efflux pump plays an important role in reduced macrolide susceptibility, accompanied by contributions from the CmeDEF efflux pump and potentially a third efflux pump. To improve clinical performance of licensed antibiotics and chemotherapeutic agents, it is important to understand the factors in Campylobacter that affect susceptibility to macrolide antibiotics. Using mutants that lack the functional genes coding for the CmeB and CmeF efflux pump proteins and the CmeR transcriptional repressor, we show that these efflux pumps are potential targets for the development of therapeutic strategies that use a combination of a macrolide with an efflux pump inhibitor (EPI) to restore macrolide efficacy.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

chelex 100树脂国产替代之路-BIOFOUNT范德生物
Chelex 100螯合离子交换树脂对铜、铁和其他重金属?的偏好显著高于对钠、钾等一价阳离子的偏好。它对二价...
2025/11/4 14:22:46

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18




 购物车
购物车 



