-
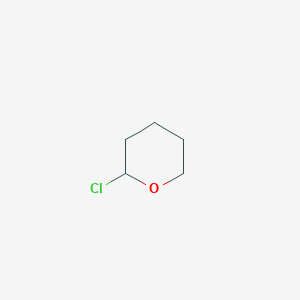
2-氯四氢吡喃
- names:
2-Chlorotetrahydro-2H-pyran
- CAS号:
3136-02-5
MDL Number: MFCD16659136 - MF(分子式): C5H9ClO MW(分子量): 120.577
- EINECS: Reaxys Number:
- Pubchem ID:524400 Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| HCC316433-50mg | 250mg | 97%药物杂质 | ¥ 2750.00 | ¥ 2750.00 | In stock | ¥ 0.00 | ||
| LSH68697-1g | 1g | 95% | ¥ 5948.00 | ¥ 5948.00 | In stock | ¥ 0.00 | ||
| HCC316433-50mg | 50mg | 97%药物杂质 | ¥ 995.00 | ¥ 995.00 | In stock | ¥ 0.00 |
| 中文别名 | 2-氯四氢吡喃(3136-02-5),2-氯-四氢-2H-吡喃 |
| 英文别名 | 2-Chlorotetrahydro-2H-pyran(3136-02-5),2-Chlorotetrahydro-2H-pyran;2-chlorotetrahydro-2H-pyran; 2-chloro-tetrahydro-2H-pyran; 2-chlorotetrahydropyran; 2-chloro-tetrahydropyran; 2H-Pyran, 2-chlorotetrahydro-; 2-chlorooxane; 2-chlor-tetrahydropyrane; 2-chlorotetrahy-dropyran |
| CAS号 | 3136-02-5 |
| SMILES | C1CCOC(C1)Cl |
| Inchi | InChI=1S/C5H9ClO/c6-5-3-1-2-4-7-5/h5H,1-4H2 |
| InchiKey | QRECIVPUECYDDM-UHFFFAOYSA-N |
| 分子式 Formula | C5H9ClO |
| 分子量 Molecular Weight | 120.577 |
| 闪点 FP | |
| 熔点 Melting point | No data available |
| 沸点 Boiling point | 155.7±33.0 °C at 760 mmHg |
| Polarizability极化度 | 11.8±0.5 10-24cm3 |
| 密度 Density | 1.1±0.1 g/cm3 |
| 蒸汽压 Vapor Pressure | 3.9±0.3 mmHg at 25°C |
| 溶解度Solubility | |
| 性状 | 棕色液体 |
| 储藏条件 Storage conditions | storage at -4℃ (1-2weeks), longer storage period at -20℃ (1-2years) |
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:2-氯四氢吡喃蒸汽压,2-氯四氢吡喃合成,2-氯四氢吡喃标准,2-氯四氢吡喃应用,2-氯四氢吡喃合成,2-氯四氢吡喃沸点,2-氯四氢吡喃闪点,2-氯四氢吡喃用途,2-氯四氢吡喃溶解度,2-氯四氢吡喃价格,2-氯四氢吡喃作用,2-氯四氢吡喃结构式,2-氯四氢吡喃用处

| 产品说明 | 2-氯四氢吡喃(2-Chlorotetrahydro-2H-pyran,3136-02-5)在SnCl4存在下,将2-氯-四氢吡喃和2,3-二氯四氢吡喃加到乙酸乙烯酯中,形成相应的宝石-乙酰氧基氯化物. |
| Introduction | 2-Chlorotetrahydro-2H-pyran(2-氯四氢吡喃,3136-02-5) add to vinyl acetate in the presence of SnCl4 |
| Application1 | The addition of 2-chlorotetrahydropyran to isopropenyl acetate is accompanied by the splitting out of HCl and the formation of the corresponding substituted enol acetate. |
| Application2 | The reaction products have been hydrolyzed to aldehydes and ketones. |
| Application3 |
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303+H313+H333 |
| 安全防护 | P264+P280+P305+P351+P338+P337+P313 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Synthesis and antileukemic activities of furanyl, pyranyl, and ribosyl derivatives of 4-(3,3-dimethyl-1-triazeno)imidazole-5-carboxamide and 3-(3,3-dimethyl-1-triazeno)pyrazole-4-carboxamide PMID 5338 |
| Reactions of alpha-acetoxy-N-nitrosopyrrolidine and alpha-acetoxy-N-nitrosopiperidine with deoxyguanosine: formation of N2-tetrahydrofuranyl and N2-tetrahydropyranyl adducts PMID 7548742; Chemical res |
| Synthesis and antileukemic activities of furanyl, pyranyl, and ribosyl derivatives of 4-(3,3-dimethyl-1-triazeno)imidazole-5-carboxamide and 3-(3,3-dimethyl-1-triazeno)pyrazole-4-carboxamide PMID 5338 |
| Nuclear quadrupole resonance. Electronic structure and stereochemistry of halogenonaphthalenones Theoretical and Experimental Chemistry 1987 |
| The stabilization of thermodynamically disfavoured conformers of chlorocyclohexane in the host lattice of tri-ortho-thymotide. Crystallographic and infrared spectroscopic studies Journal of inclusion |
Abstract:From the reaction of silylated 4-(3,3-dimethyl-1-triazeno)imidazole-5-carboxamide (DTIC, 5) and 3-(3,3-dimethyl-1-triazeno)pyrazole-4-carboxamide (DTPC, 9) with 2-chlorotetrahydrofuran, we have isolated in both cases a single tetrahydrofuran-2-yl derivative. However, when silylated DTPC was reacted with 2-chlorotetrahydropyran, two tetrahydropyran-2-yl compounds were obtained, and these were shown to be positional isomers on the basis of 1H NMR and UV data. These furanyl and pyranyl derivatives were tested for antileukemic activity (L-1210, in vivo) and the results were compared with the results obtained for the corresponding ribosyl derivatives of DTIC and DTPC.
2.Reactions of alpha-acetoxy-N-nitrosopyrrolidine and alpha-acetoxy-N-nitrosopiperidine with deoxyguanosine: formation of N2-tetrahydrofuranyl and N2-tetrahydropyranyl adducts/PMID 7548742; Chemical research in toxicology 1995 Jun; 8(4):607-16/Name matches: 2-chlorotetrahydrofuran 2-chloro-3,4,5,6-tetrahydro-2h-pyran
Abstract:The goal of this study was to compare the reactions of alpha-acetoxy-N-nitrosopyrrolidine (alpha-acetoxyNPYR) and alpha-acetoxy-N-nitrosopiperidine (alpha-acetoxyNPIP) with deoxyguanosine (dG). alpha-AcetoxyNPYR and alpha-acetoxyNPIP are stable precursors to the alpha-hydroxynitrosamines which are formed metabolically from NPYR and NPIP. These alpha-hydroxynitrosamines are believed to be the proximate carcinogens of NPYR and NPIP. NPYR and NPIP, although structurally similar, have remarkably different carcinogenic properties, and a comparison of the reactions of their metabolically activated forms with dG and ultimately DNA could provide insights on their mechanisms of carcinogenicity. Reactions of alpha-acetoxyNPYR and alpha-acetoxyNPIP with dG were carried out at 37 degrees C and pH 7.0. The products were analyzed by HPLC and characterized by their spectral properties and by comparison to standards. In each reaction, one of the major products was a new type of dG adduct: N2-(tetrahydrofuran-2- yl)dG (THF-dG) from alpha-acetoxyNPYR and N2-(3,4,5,6-tetrahydro-2H-pyran-2-yl)dG (THP-dG) from alpha-acetoxyNPIP. THF-dG was synthesized independently by reaction of either 2-chlorotetrahydrofuran or 2,3-dihydrofuran with dG. Similarly, THP-dG was prepared by reaction of 2-chloro-3,4,5,6-tetrahydro-2H-pyran with dG. The structures of THF-dG and THP-dG were established by their UV and 1H-NMR spectra. THF-dG was less stable than THP-dG, but could be readily converted to a stable derivative, N2-(4-hydroxybutyl)dG, by reaction with NaBH4. THF-dG and THP-dG were converted to dG and 2-hydroxytetrahydrofuran or 2-hydroxy-3,4,5,6-tetrahydro-2H-pyran, respectively, upon neutral thermal or acid hydrolysis. This reaction was found to be reversible, with the adducts being produced in substantial amounts by reaction of 2-hydroxytetrahydrofuran or 2-hydroxy-3,4,5,6-tetrahydro-2H-pyran with dG. The latter reaction accounts for part of the THF-dG and THP-dG produced from the alpha-acetoxynitrosamines; stable oxonium ion-derived electrophiles may also be involved in the formation of THF-dG and THP-dG. Comparisons of the yields of various adducts in the reaction of alpha-acetoxyNPYR and alpha-acetoxyNPIP with dG showed some major differences. Whereas yields of THF-dG and THP-dG were similar, adducts formed from open chain diazonium ion or related intermediates were formed more extensively from alpha-acetoxyNPYR than from alpha-acetoxyNPIP. Adducts formed from enal products of the two nitrosamines were also different. Adduct formation as characterized in this study may account for some of the contrasting carcinogenic properties of NPYR and NPIP.
3.Studies in the field of oxygen-containing heterocycles Chemistry of Heterocyclic Compounds 1973
Abstract:2-Chloro- and 2,3-dichlorotetrahydropyrans add to vinyl acetate in the presence of SnCl4 with the formation of the corresponding gem-acetoxychlorides. The addition of 2-chlorotetrahydropyran to isopropenyl acetate is accompanied by the splitting out of HCl and the formation of the corresponding substituted enol acetate. The reaction products have been hydrolyzed to aldehydes and ketones.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
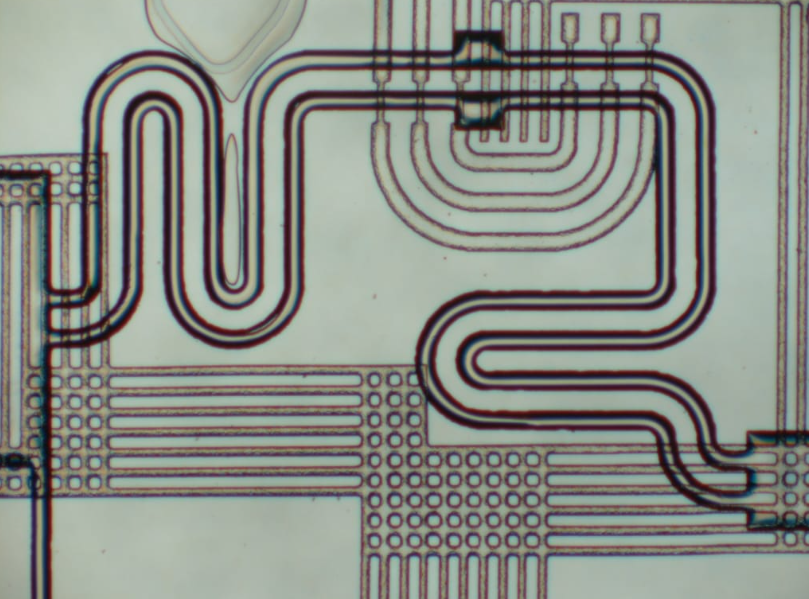
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18

牛血清白蛋白(BSA)常见问题
牛血清白蛋白(BSA)常见问题:牛血清白蛋白(BSA)在实验室中是通用的,可用于蛋白质印迹、细胞组织培养、P...
2022/10/19 9:39:51




 购物车
购物车 



