-
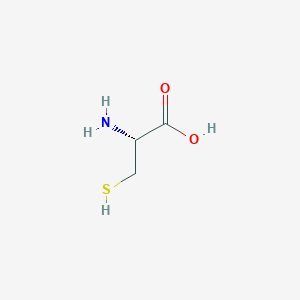
L-半胱氨酸
NMR and HPLC COA下载 MSDS下载 - Names:
L-Cysteine
- CAS号:
52-90-4
MDL Number: MFCD00064306 - MF(分子式): HSCH2CH(NH2)CO2H MW(分子量): 121.16
- EINECS:200-158-2 Reaxys Number:
- Pubchem ID: Brand:BIOFOUNT
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| SS3295-500g | 500g | 99% | ¥ 290.00 | ¥ 290.00 | 3-5days | ¥ 0.00 | ||
| SS3295-100g | 100g | 99% | ¥ 102.00 | ¥ 102.00 | Instock | ¥ 0.00 | ||
| SS3295-25g | 25g | 99% | ¥ 58.00 | ¥ 58.00 | Instock | ¥ 0.00 |
| 中文别名 | L-半胱氨酸(CAS:52-90-4); L-beta-巯基丙氨酸; L-2-氨基-3-巯基丙酸; L-半膀胱氨基酸; L-半胱氨酸碱; L-丰胱胺酸; L-硫氢化氨基丙酸; 半胱氨酸; 一巯基氨基丙盐酸盐; L-β-硫氢代丙氨酸; (+)-2-氨基-3-巯基丙酸; L-Β-巯基丙氨酸; Α-氨基-Β-巯基丙酸; 巯基丙氨酸; L-低铁-半胱氨酸盐酸盐—水物; L-半光氨酸 L-CYSTEINE; Β-巯基-Α-氨基丙酸; L(+)-半胱氨酸/L(+)-巯基氨基丙酸 |
| 英文别名 | L-Cysteine(CAS:52-90-4);L-cysteine; cysteine; Cystein; Half-cystine;; (R)-Cysteine Thioserine; (R)-2-Amino-3-mercaptopropanoic acid; L-(+)-Cysteine; L-Cystein |
| CAS号 | 52-90-4 |
| SMILES | C([C@@H](C(=O)O)N)S |
| Inchi | InChI=1S/C3H7NO2S/c4-2(1-7)3(5)6/h2,7H,1,4H2,(H,5,6)/t2-/m0/s1 |
| InchiKey | XUJNEKJLAYXESH-REOHCLBHSA-N |
| 分子式 Molecular Weight | HSCH2CH(NH2)CO2H |
| 分子量 Formula | 121.16 |
| 闪点 FP | |
| 熔点 Melting point | 220°C |
| 沸点 Boiling point | 293.9±35.0 °C at 760 mmHg |
| Polarizability极化度 | 11.5±0.5 10-24cm3 |
| 密度 Density | 1.3±0.1 g/cm3 |
| 蒸汽压 Vapor Pressure | 0.0±1.3 mmHg at 25°C |
| 溶解度Solubility | Soluble in water (10 mg/ml), 1N HCl (50 mg/ml), and ethanol. |
| 性状 | 8.75 o (C=12, 2N HCL) |
| 储藏条件 Storage conditions | 充氩储存;储存温度2-8℃ |
| 动物 | 测试类型 | 途径 | 实验摄入量 (标准摄入量) | 影响 | 文献来源 |
| rat | LD50 | oral | 1890 mg/kg (1890 mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); LUNGS, THORAX, OR RESPIRATION: DYSPNEA; KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION | Agents and Actions, A Swiss Journal of Pharmacology., 4(125), 1974 [PMID:4842541] |
| rat | LD50 | intraperitoneal | 1620 mg/kg (1620 mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: ATAXIA; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics., 7(1251), 1973 |
| rat | LD50 | subcutaneous | 1550 mg/kg (1550 mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: ATAXIA; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics., 7(1251), 1973 |
| rat | LD50 | intravenous | 1140 mg/kg (1140 mg/kg) | Journal of the American College of Toxicology., 12(113), 1993 | |
| mouse | LD50 | oral | 660 mg/kg (660 mg/kg) | Archives of Toxicology., 41(79), 1978 [PMID:214056] | |
| mouse | LD50 | intraperitoneal | 1400 mg/kg (1400 mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: ATAXIA; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics., 7(1251), 1973 |
| mouse | LD50 | subcutaneous | 1360 mg/kg (1360 mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY); BEHAVIORAL: ATAXIA; LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Oyo Yakuri. Pharmacometrics., 7(1251), 1973 |
| mouse | LD50 | intravenous | 1250 mg/kg (1250 mg/kg) | Yakugaku Zasshi. Journal of Pharmacy., 89(1138), 1969 [PMID:5388616] |
L-半胱氨酸(CAS:52-90-4;英文名:L-Cysteine;)实验注意事项:
1.使用52-90-4实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.使用52-90-4实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害。
3.取样品52-90-4的移液枪头需及时更换,必要时为避免交叉污染尽可能选择滤芯吸头。
4.称量药品时选用称量纸,并无风处取药和称量以免扬撒,试剂的容器使用前务必确保干净,并消毒。
5.取药品52-90-4时尽量采用多个药勺分别使用,使用后清洗干净。
6.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染。
大规格定制:定制产品请将信息发送至sales@bio-fount.com。
Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tag:L-半胱氨酸蒸汽压,L-半胱氨酸合成,L-半胱氨酸标准,L-半胱氨酸应用,L-半胱氨酸合成,L-半胱氨酸沸点,L-半胱氨酸闪点,L-半胱氨酸用途,L-半胱氨酸溶解度,L-半胱氨酸价格,L-半胱氨酸作用,L-半胱氨酸结构式,L-半胱氨酸用处

| 产品说明 | L-半胱氨酸(52-90-4)也称为C或E920,L-半胱氨酸属于称为半胱氨酸及其衍生物的有机化合物,L-半胱氨酸溶解度,L-半胱氨酸MSDS,L-半胱氨酸结构式详见主页. |
| Introduction | L-Cysteine(L-半胱氨酸,52-90-4), also known as C or E920, belongs to the class of organic compounds known as cysteine and derivatives. |
| Application1 | Cysteine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen |
| Application2 | A non-essential amino acid. |
| Application3 | L-半胱氨酸以固体,可溶(在水中)和中等酸性化合物(基于其pKa)存在。 |
2.L-cysteine is an optically active form of cysteine having L-configuration. It has a role as a flour treatment agent, a human metabolite and an EC 4.3.1.3 (histidine ammonia-lyase) inhibitor. It is a serine family amino acid, a proteinogenic amino acid, a cysteine and a L-alpha-amino acid. It is a conjugate base of a L-cysteinium. It is a conjugate acid of a L-cysteinate(1-). It is an enantiomer of a D-cysteine. It is a tautomer of a L-cysteine zwitterion.
3.Cysteine is a non-essential sulfur-containing amino acid in humans, related to cystine, Cysteine is important for protein synthesis, detoxification, and diverse metabolic functions. Found in beta-keratin, the main protein in nails, skin, and hair, Cysteine is important in collagen production, as well as skin elasticity and texture. Also required in the manufacture of amino acid taurine, Cysteine is a component of the antioxidant glutathione, and plays a role in the metabolism of essential biochemicals such as coenzyme A, heparin, and biotin.
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not Available |
| 安全声明 | H302 |
| 安全防护 | |
| 备注 | 避免吸入,误食以及与皮肤接触 |
| 31444509 2019-10-01 Microsomal prostaglandin E synthase 2 deficiency is resistant to acetaminophen-induced liver injury Archives of toxicology |
| 31465224 2019-09-26 Development of Novel Irreversible Pyruvate Kinase M2 Inhibitors Journal of medicinal chemistry |
| 31400341 2019-09-25 Opposite clozapine and ziprasidone effects on the reactivity of plasma albumin SH-group are the consequence of their different binding properties dependent on protein fatty acids c |
| 31067004 2019-08-01 Association of p38MAPK-p53-Fas aggregation in S-allyl cysteine mediated regulation of hepatocarcinoma Environmental toxicology |
| 30242128 2018-11-16 Dynamic disulfide exchange in a crystallin protein in the human eye lens promotes cataract-associated aggregation The Journal of biological chemistry |
Abstract in English, Russian:Mass spectrometric proteomic analysis at the sample preparation stage involves the artificial reduction of disulfide bonds in proteins formed between cysteine residues. Such bonds, when preserved in their native state, complicate subsequent enzymatic hydrolysis and interpretation of the research results. To prevent the re-formation of the disulfide bonds, cysteine residues are protected by special groups, most often by alkylation. In this review, we consider the methods used to modify cysteine residues during sample preparation, as well as possible artifacts of this stage. Particularly, adverse reactions of the alkylating agents with other amino acid residues are described. The most common alkylating compound used to protect cysteine residues in mass spectrometric proteomic analysis is iodoacetamide. However, an analysis of the literature in this area indicates that this reagent causes more adverse reactions than other agents used, such as chloroacetamide and acrylamide. The latter can be recommended for wider use. In the review we also discuss the features of the cysteine residue modifications and their influence on the efficiency of the search for post-translational modifications and protein products of single nucleotide substitutions.
2.Cysteine Oxidations in Mitochondrial Membrane Proteins: The Case of VDAC Isoforms in Mammals/PMID 32582695; Frontiers in cell and developmental biology 2020; 8(?):397 (Review Article)/Name matches: disulfide cysteine
Abstract:Cysteine residues are reactive amino acids that can undergo several modifications driven by redox reagents. Mitochondria are the source of an abundant production of radical species, and it is surprising that such a large availability of highly reactive chemicals is compatible with viable and active organelles, needed for the cell functions. In this work, we review the results highlighting the modifications of cysteines in the most abundant proteins of the outer mitochondrial membrane (OMM), that is, the voltage-dependent anion selective channel (VDAC) isoforms. This interesting protein family carries several cysteines exposed to the oxidative intermembrane space (IMS). Through mass spectrometry (MS) analysis, cysteine posttranslational modifications (PTMs) were precisely determined, and it was discovered that such cysteines can be subject to several oxidization degrees, ranging from the disulfide bridge to the most oxidized, the sulfonic acid, one. The large spectra of VDAC cysteine oxidations, which is unique for OMM proteins, indicate that they have both a regulative function and a buffering capacity able to counteract excess of mitochondrial reactive oxygen species (ROS) load. The consequence of these peculiar cysteine PTMs is discussed.
3.Cysteine, glutathione and a new genetic code: biochemical adaptations of the primordial cells that spread into open water and survived biospheric oxygenation/PMID 31318686; Biological chemistry 2020 02; 401(2):213-231 (Review Article)/Name matches: glutathione cysteine
Abstract:Life most likely developed under hyperthermic and anaerobic conditions in close vicinity to a stable geochemical source of energy. Epitomizing this conception, the first cells may have arisen in submarine hydrothermal vents in the middle of a gradient established by the hot and alkaline hydrothermal fluid and the cooler and more acidic water of the ocean. To enable their escape from this energy-providing gradient layer, the early cells must have overcome a whole series of obstacles. Beyond the loss of their energy source, the early cells had to adapt to a loss of external iron-sulfur catalysis as well as to a formidable temperature drop. The developed solutions to these two problems seem to have followed the principle of maximum parsimony: Cysteine was introduced into the genetic code to anchor iron-sulfur clusters, and fatty acid unsaturation was installed to maintain lipid bilayer viscosity. Unfortunately, both solutions turned out to be detrimental when the biosphere became more oxidizing after the evolution of oxygenic photosynthesis. To render cysteine thiol groups and fatty acid unsaturation compatible with life under oxygen, numerous counter-adaptations were required including the advent of glutathione and the addition of the four latest amino acids (methionine, tyrosine, tryptophan, selenocysteine) to the genetic code. In view of the continued diversification of derived antioxidant mechanisms, it appears that modern life still struggles with the initially developed strategies to escape from its hydrothermal birthplace. Only archaea may have found a more durable solution by entirely exchanging their lipid bilayer components and rigorously restricting cysteine usage.
Ren 化学品安全技术说明书 | 版本:1.0 | |||
按照GB/T16483、GB/T17519编制 | 修订日期:10.07.2019 | |||
打印日期:19.02.2020 | ||||
版权所有:范德(北京)生物科技有限责任公司 | 最初编制日期:25.05.2017 | |||
公司网站:WWW.BIO-FOUNT.COM | SDS编号:BIOFOUNT-SS3295 | |||
版权所有:BIOFOUNT BEIJING BIO TECH CO.,LTD | 产品编号:SS3295 | |||
L-半胱氨酸 | ||||
说明书目录 | ||||
第1部分 | 第2部分 | 危险性概述 | ||
第3部分 | 成分/组成信息 | 第4部分 | 急救措施 | |
第5部分 | 消防措施 | 第6部分 | 泄露应急处理 | |
第7部分 | 操作处置与储存 | 第8部分 | 接触控制/个体防护 | |
第9部分 | 理化性质 | 第10部分 | 稳定性和反应性 | |
第11部分 | 毒理学信息 | 第12部分 | 生态学危害信息 | |
第13部分 | 废弃处置 | 第14部分 | 运输信息 | |
第15部分 | 法律法规信息 | 第16部分 | 其他补充信息 | |
第1部分:化学品及企业标识 | ||||
1.1 产品标识 | ||||
L-半胱氨酸 | ||||
ENGLISH NAME: | L-Cysteine | |||
SS3295 | ||||
BIOFOUNT | ||||
52-90-4 | ||||
1.2 安全技术说明书提供者的详情 | ||||
制造商或供应商名称: | ||||
制造地址: | 59 KANGTAI AVENUE BINHAI NEW DISTRICT TIANJIN 300450 TIANJIN CHINA 范德(天津)生物科技有限责任公司 天津市滨海新区康泰大道59号九州通绿谷健康产业园 邮政编码:300450 | |||
电话号码: | ||||
1.3 应急咨询电话 | ||||
紧急联系电话: | ||||
1.4 物质或混合物的推荐用途和限制用途 | ||||
已确认的各用途: | 仅用于科学研发,不作为药品、家庭或其它用途。 | |||
第2部分:危险性概述 | ||||
2.1 GHS危险性类别 | ||||
暂无数据 | ||||
2.2 GHS 标签要素,包括防范说明 | ||||
象形图 | ||||
暂无数据 | ||||
Warning | ||||
H302 | ||||
警告申明 | ||||
避免吸入,误食以及与皮肤接触 | ||||
暂无数据 | ||||
事故响应 | ||||
1.化学品使用过程中,当出现事故或者有紧急情况发生时,当事人应第一时间向应急小组负责人汇报后,由应急小组采取措施防止事态扩大。2.应急小组对受害人采取救护措施。 | ||||
充氩储存;储存温度2-8℃ | ||||
废弃处置 | ||||
暂无数据 | ||||
2.3 物理和化学危险 | ||||
暂无数据 | ||||
2.4 健康危害 | ||||
暂无数据 | ||||
2.5 环境危害 | ||||
暂无数据 | ||||
2.6 其它危害物 | ||||
暂无数据 | ||||
第3部分:成分/组成信息 | ||||
物质/混合物 | 暂无数据 | |||
3.1 物 质 | ||||
HSCH2CH(NH2)CO2H | ||||
121.16 | ||||
110-91-8 | ||||
EC-编号 | 暂无数据 | |||
根据相应法规,无需披露具体组份。 | ||||
第4部分:急救措施 | ||||
4.1 必要的急救措施描述 | ||||
吸入 | ||||
立即将患者移至空气新鲜处,发现呼吸困难时,必须立即采取吸氧处理,停止呼吸时采取人工呼吸。同时联系及时就医。 | ||||
皮肤接触 | ||||
立即脱去或者剪去污染的衣物,迅速用大量的流动清水冲10-20分钟甚至更长时间后,赴医院就医。 | ||||
眼睛接触 | ||||
立即用大量的流动清水冲10-20分钟后赴医院就医处理。 | ||||
食入 | ||||
误食化学物品后,应立即采取措施进行催吐。1.若误食化学品呈酸性,则可服用大量牛奶和水,促使食如折呕吐。2.若误食化学品呈碱性,则可服用大量牛奶、清水和醋,促使其呕吐,紧急处理后,应及时送至医院进行治疗(仅供参考)。食如者昏迷状态下禁止催吐,以免造成窒息。 | ||||
4.2 最重要的症状和健康影响 | ||||
最重要的已知症状及作用已在标签(参见章节2.2)和/或章节11中介绍 | ||||
暂无数据 | ||||
4.4 对医生的特别提示 | ||||
暂无数据 | ||||
第5部分:消防措施 | ||||
5.1 灭火介质 | ||||
采用泡沫灭火器、二氧化碳灭火器,避免造成二次污染发生。 | ||||
5.2 源于此物质或混合物的特别的危害 | ||||
暂无数据 | ||||
5.3 灭火注意事项及保护措施 | ||||
小规模着火需戴好口罩,防止有毒气体吸入。火灾发生时及时启动应急相应系统撤离至上风口处,并联系当地消防部门灭火。 | ||||
第6部分:泄露应急处理 | ||||
1.泄露后首先启动应急相应系统2.泄露处理前,需穿戴好安全安全防护鞋、穿戴好安全防护手套(强酸性物质需穿戴防酸碱手套)、根据吸入危险性穿戴相应防护面罩。 有关个人防护,请看第8部分。 | ||||
6.2 环境保护措施 | ||||
参照《范德生物化学废弃物处理方法》处理,防止对环境造成危害,处理后交由有资质的废弃物处理结构进行处理,以免造成环境污染。 | ||||
参照《范德生物化学品废弃物处理方法》对泄露的化学品进行处理,处理前需用化学品吸附岩棉对泄露区域进行围挡,形成“围堰”防止泄露扩大。 | ||||
6.4 参考其他部分 | ||||
丢弃处理请参阅第13节。 | ||||
第7部分:操作处置与储存 | ||||
7.1 安全操作的注意事项 | ||||
使用过程请穿戴好口罩,手套等防护用品,避免与皮肤接触、吸入、误食危险。 有关预防措施,请参见章节2.2。 | ||||
7.2 安全储存的条件,包括任何不兼容性 | ||||
暂时无法提供详细数据,尽可能避免与其他化合物混合存储,避光、通风处存储。 | ||||
第8部分:接触控制/个体防护 | ||||
8.1 控制参数 | ||||
暂无数据 | ||||
8.2 暴露控制 | ||||
适当的技术控制 | ||||
暂无数据 | ||||
个体防护装备 | ||||
一般情况下穿戴安全防护眼镜即可,如有飞溅液体、粉末产生时,请佩戴防溅面罩进行防护。穿戴的防护用品需取得如:GB、NIOSH (美国) 或 EN 166(欧盟) 等相关认证。 | ||||
手套脱去注意事项:手套在使用前必须进行检查,请使用正确的方法脱除手套(不接触手套外部表面),避免身体任何皮肤部位接触到此产品。根据相关法律法规和实验室管理规范制度,手套使用过后,请将被污染的手套谨慎处理,工作后清洗并吹干双手。 所选择的保护手套必须符合法规《劳动防护用品配备标准》、(EU)2016/425以及从此类法规衍生出来的EN 374标准规范。 完全接触保护要求: 手套材料:丁腈橡胶 手套最小的层厚度:0.11 MM 手套溶剂渗透时间:480 分钟 飞溅保护要求: 材料:丁腈橡胶 最小的层厚度 0.11 MM 溶剂渗透时间:480 分钟 如果以溶剂形式应用或与其它物质混合应用,或在不同于《劳动防护用品配备标准》,EN 374规定的条件下应用,请与EC批准的手套的供应商联系。该条只是作为推荐性建议,如遇特殊情况,务必请熟悉该产品属性的专家,选取相关防护用品。此条建议不应该被认定为适应所有特殊条件防护,请根据所处工作条件请求专业工程师指导采取相应防护措施。 | ||||
选择身体部分的防护措施,需要根据危险物质的类型、浓度、量以及特定的工作环境。身体部分防护设备、防护服的类型,必须根据使用者工作场所中的危险物质的浓度、数量进行选择。 | ||||
一般情况下穿戴普通的医用口罩保护呼吸系统即可。有酸雾产生式活性炭类口罩起不到防护作用,如需对粉尘造成损害进行防护时,请采用N95型(US)或P1型(EN 143)类口罩或者防尘面具。特殊情况下使用自吸式呼吸器时,使用的呼吸器必须对呼吸器密闭性、空气供应系统、供气压进行测试,当然呼吸器需通过强制认证标准如:GB、NIOSH(US)、CEN(EU)。 | ||||
环境暴露的控制 | ||||
不要让产品进入下水道。 | ||||
第9部分:理化特性 | ||||
9.1 基本的理化特性的信息 | ||||
形状:暂无数据 | ||||
颜色:暂无数据 | ||||
气味 | 暂无数据 | |||
气味阈值 | 暂无数据 | |||
暂无数据 | ||||
220°C | ||||
初沸点和沸程 | 293.9±35.0 °C at 760 mmHg | |||
闪点 | 131.5±25.9 °C | |||
蒸发速率 | 暂无数据 | |||
易燃性(固体,气体) | 暂无数据 | |||
高的/低的燃烧性或爆炸性限度 | 暂无数据 | |||
蒸气压 | 0.0±1.3 mmHg at 25°C | |||
蒸气焓 | 58.7±6.0 kJ/mol | |||
密度/相对密度 | 1.3340 | |||
Soluble in water (10 mg/ml), 1N HCl (50 mg/ml), and ethanol. | ||||
正辛醇/水分配系数 | Log Kow (KOWWIN v1.67 estimate) = -3.05
Log Kow (Exper. database match) = -2.49
Exper. Ref: Hansch,C et al. (1995)/ Boiling Pt, Melting Pt, Vapor Pressure Estimations (MPBPWIN v1.42):
Boiling Pt (deg C): 348.64 (Adapted Stein & Brown method)
Melting Pt (deg C): 199.27 (Mean or Weighted MP)
VP(mm Hg,25 deg C): 6.73E-007 (Modified Grain method)
MP (exp database): 240 dec deg C
Subcooled liquid VP: 0.000142 mm Hg (25 deg C, Mod-Grain method) | |||
正辛醇空气分配系数 | Log Kow used: -2.49 (exp database)
Log Kaw used: -8.664 (HenryWin est)
Log Koa (KOAWIN v1.10 estimate): 6.174
Log Koa (experimental database): None | |||
自燃温度 | 暂无数据 | |||
分解温度 | 暂无数据 | |||
黏度 | 暂无数据 | |||
暂无数据 | ||||
氧化性 | 暂无数据 | |||
根据碎片估算水溶胶 | Wat Sol (v1.01 est) = 1.5081e+005 mg/L
Wat Sol (Exper. database match) = 277000.00
Exper. Ref: BEILSTEIN | |||
亨利定律常数(25摄氏度) | Bond Method : 5.30E-011 atm-m3/mole
Group Method: Incomplete
Henrys LC [VP/WSol estimate using EPI values]: 9.477E-013 atm-m3 | |||
9.2 其他安全信息 | ||||
暂无数据 | ||||
第10部分:稳定性和反应性 | ||||
10.1 稳定性 | ||||
暂无数据 | ||||
10.2 危险反应 | ||||
暂无数据 | ||||
10.3 应避免的条件 | ||||
暂无数据 | ||||
10.4 禁配物 | ||||
强氧化剂 | ||||
10.5 危险的分解产物 | ||||
暂无数据 | ||||
第11部分:毒理学信息 | ||||
11.1 毒理学影响信息 | ||||
暂无数据 | ||||
皮肤腐蚀/刺激 | ||||
暂无数据 | ||||
暂无数据 | ||||
呼吸或皮肤过敏 | ||||
暂无数据 | ||||
暂无数据 | ||||
暂无数据 | ||||
生殖毒性 | ||||
暂无数据 | ||||
特异性靶器官系统毒性(一次接触) | ||||
暂无数据 | ||||
特异性靶器官系统毒性(反复接触) | ||||
暂无数据 | ||||
吸入危害 | ||||
暂无数据 | ||||
附加说明 | ||||
暂无数据 | ||||
第12部分:生态学危害信息 | ||||
12.1 生态毒性 | ||||
暂无数据 | ||||
12.2 持久性和降解性 | ||||
暂无数据 | ||||
12.3 快速生物降解的可能性 | ||||
Biowin1 (Linear Model) : 0.9164
Biowin2 (Non-Linear Model) : 0.9544 | ||||
12.4 专家调查生物降解结果 | ||||
Biowin3 (Ultimate Survey Model): 3.3205 (days-weeks )
Biowin4 (Primary Survey Model) : 4.1018 (days ) | ||||
12.5 MITI生物降解的可能性 | ||||
Biowin5 (MITI Linear Model) : 0.5649
Biowin6 (MITI Non-Linear Model): 0.5518 | ||||
12.6 厌氧生物降解的可能性 | ||||
Biowin7 (Anaerobic Linear Model): 1.0603 | ||||
12.7 现成的生物降解性预测 | ||||
YES | ||||
12.8 碳氢化合物生物降解 | ||||
Structure incompatible with current estimation method! | ||||
12.9 对气溶胶的吸附 | ||||
Vapor pressure (liquid/subcooled): 0.0189 Pa (0.000142 mm Hg)
Log Koa (Koawin est ): 6.174
Kp (particle/gas partition coef. (m3/ug)):
Mackay model : 0.000158
Octanol/air (Koa) model: 3.66E-007 | ||||
12.10 羟基自由基反应 | ||||
OVERALL OH Rate Constant = 79.6245 E-12 cm3/molecule-sec
Half-Life = 0.134 Days (12-hr day; 1.5E6 OH/cm3)
Half-Life = 1.612 Hrs | ||||
12.11 臭氧反应 | ||||
No Ozone Reaction Estimation | ||||
12.12 空气中颗粒物吸附的分数(PHI) | ||||
0.0091 (Junge,Mackay)
Note: the sorbed fraction may be resistant to atmospheric oxidation | ||||
12.13 土壤吸附系数 | ||||
暂无数据 | ||||
12.14 碱/酸催化水解(25℃) | ||||
Koc : 2.75
Log Koc: 0.439 / Aqueous Base/Acid-Catalyzed Hydrolysis (25 deg C) [HYDROWIN v1.67]:
Rate constants can NOT be estimated for this structure!/ | ||||
12.15 利用对数KOW估算生物累积量 | ||||
Log BCF from regression-based method = 0.500 (BCF = 3.162)
log Kow used: -2.49 (expkow database) | ||||
12.16 废水处理中的去除 | ||||
Total removal: 1.85 percent
Total biodegradation: 0.09 percent
Total sludge adsorption: 1.75 percent
Total to Air: 0.00 percent
(using 10000 hr Bio P,A,S) | ||||
12.17 三级逸度模型 | ||||
Mass Amount Half-Life Emissions
(percent) (hr) (kg/hr)
Air 0.00191 3.22 1000
Water 34.5 208 1000
Soil 65.4 416 1000
Sediment 0.0597 1.87e+003 0
Persistence Time: 386 hr | ||||
12.18 土壤中的迁移性 | ||||
暂无数据 | ||||
12.19 PBT和VPVB的结果评价 | ||||
暂无数据 | ||||
12.20 其他环境有害作用 | ||||
暂无数据 | ||||
第13部分:废弃处置 | ||||
13.1 废物处理 | ||||
None | ||||
None | ||||
第14部分:运输信息 | ||||
14.1 联合国编号 / UN NUMBER | ||||
欧洲陆运危规 / ER/RID: | None | |||
国际海运危规 / IMDG: | None | |||
国际空运危规 / IATA-DGR: | None | |||
14.2 联合国运输名称 / UN PROPER SHIPPING NAME | ||||
欧洲陆运危规: | None | |||
国际海运危规: | None | |||
国际空运危规: | None | |||
欧洲陆运危规 / ER/RID: | None | |||
国际海运危规 / IMDG: | None | |||
国际空运危规 / IATA-DGR: | None | |||
欧洲陆运危规 / ER/RID : | None | |||
国际海运危规 / IM0DG: | None | |||
国际空运危规 / IATA-DGR: | None | |||
None | ||||
14.6 特殊防范措施 / SPECIAL PRECAUTIONS FOR USER | ||||
None | ||||
None | ||||
第15部分:法律法规信息 | ||||
适用法规 | ||||
《中华人民共和国安全生产法》、《职业病防治法》、《化学化工实验室安全管理规范》 | ||||
其它的规定 | ||||
《生产安全事故报告和调查处理条例》、《职业病防治法》、《职业安全和卫生法》美国1970 | ||||
第16部分:其他补充信息 | ||||
其他信息 版权所有:BIOFOUNT BEIJING BIO TECH CO.,LTD 公司。许可无限制纸张拷贝,仅限于内部使用。 上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。BIOFOUNT公司及其附属公司对任何操作或者接触上述产品而引起的损害不负有任何责任。更多使用条款,参见发票或包装条的反面。 更多销售条款及条件请参见HTTP://WWW.BIO-FOUNT.COM/或发票或装箱单的背面。欲悉详情,请联系:SALES@BIO-FOUNT.COM | ||||
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
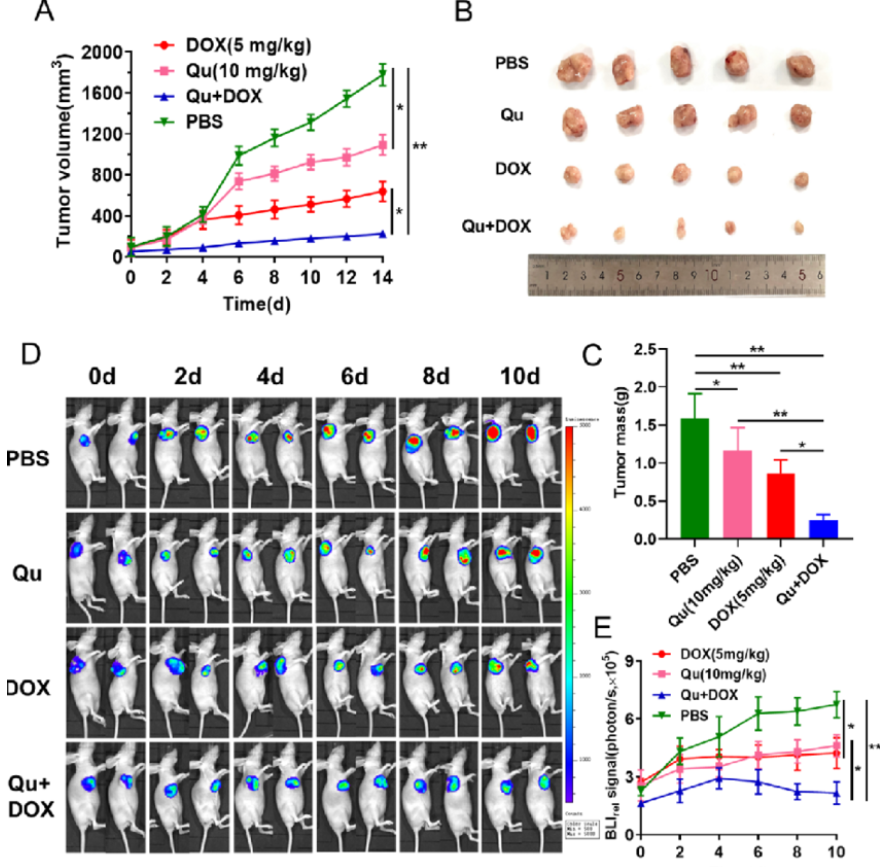
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18

牛血清白蛋白(BSA)常见问题
牛血清白蛋白(BSA)常见问题:牛血清白蛋白(BSA)在实验室中是通用的,可用于蛋白质印迹、细胞组织培养、P...
2022/10/19 9:39:51




 购物车
购物车 



