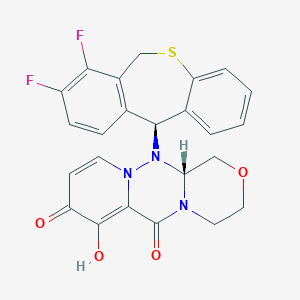
-
巴洛沙韦
NMR and HPLC COA下载 MSDS下载 - Names:
Baloxavir
- CAS号:
1985605-59-1
MDL Number: MFCD31619273 - MF(分子式): C26H21F2NO4S MW(分子量): 481.51
- EINECS: Reaxys Number:
- Pubchem ID: Brand:BIOFOUNT
巴洛沙韦(1985605-59-1,Baloxavir,Baloxavir acid,S-033447)是流感帽依赖性核酸内切酶的抑制剂,巴洛沙韦可用于治疗A型和B型流感。Baloxavir一次性使用一次,与血清酶升高或临床上明显的肝损伤无关。巴洛沙韦用于治疗甲型和乙型流感病毒感染的流感帽依赖性核酸内切酶的选择性抑制剂。
| 货品编码 | 规格 | 纯度 | 价格 (¥) | 现价(¥) | 特价(¥) | 库存描述 | 数量 | 总计 (¥) |
|---|---|---|---|---|---|---|---|---|
| YZM000725-5mg | 5mg | 99.71% | ¥ 11137.00 | ¥ 11137.00 | 2-3天 | ¥ 0.00 | ||
| YZM000725-1mg | 1mg | 99.71% | ¥ 4387.50 | ¥ 4387.50 | 2-3天 | ¥ 0.00 | ||
| HCC307873-0.5mg | 0.5mg | 97% | ¥ 4655.00 | ¥ 4655.00 | 4-7周 | ¥ 0.00 |
| 中文别名 | 巴洛沙韦(1985605-59-1);巴洛沙韦酸;S-033447;(12aR)-12-[(11S)-7,8-二氟-6,11-二氢二苯并[b,e]噻庚英-11-基]-6,8-二氧代-3,4,6,8,12,12a-六氢-1H-[1,4]噁嗪并[3,4-c]吡啶并[2,1-f][1,2,4]三嗪-7-醇; |
| 英文别名 | Baloxavir(1985605-59-1);Baloxavir acid; BXA; Xofluza; (((12aR)-12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-6,8-dioxo-3,4,6,8,12,12ahexahydro-1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazin-7-yl)oxy)methyl methyl carbonate;10.1H-(1,4)oxazino(3,4-C)pyrido(2,1-F)(1,2,4)triazine-6,8-dione, 12-((11S)-7,8-difluoro-6,11-dihydrodibenzo(b,E)thiepin-11-yl)-3,4,12,12a-tetrahydro-7-hydroxy-, (12aR)-;baloxavir;baloxavir marboxil;Xofluza; |
| CAS号 | 1985605-59-1 |
| SMILES | C1COCC2N1C(= O)C3 = C(C(= O)C = CN3N2C4C5 = C(CSC6 = CC = CC = C46)C(= C(C = C5)F)F)O |
| Inchi | nChI = 1S / C24H19F2N3O4S / c25-16-6-5-13-15(20(16)26)12-34-18-4-2-1-3-14(18)21(13)29-19- 11-33-10-9-27(19)24(32)22-23(31)17(30)7-8-28(22)29 / h1-8,19,21,31H,9-12H2 / t19-,21 + / m1 / s1 |
| InchiKey | FIDLLEYNNRGVFR-CTNGQTDRSA-N |
| 分子式 Molecular Weight | C26H21F2NO4S |
| 分子量 Formula | 481.51 |
| 闪点 FP | |
| 熔点 Melting point | Not available |
| 沸点 Boiling point | 644.7±65.0 °C at 760 mmHg |
| Polarizability极化度 | Not available |
| 密度 Density | 1.6±0.1 g/cm3 |
| 蒸汽压 Vapor Pressure | 0.0±2.0 mmHg at 25°C |
| 溶解度Solubility | 溶于DMSO |
| 性状 | 固体粉末 |
| 储藏条件 Storage conditions | storage at -4℃ (1-2weeks), longer storage period at -20℃ (1-2years) |
巴洛沙韦(1985605-59-1,Baloxavir,Baloxavir acid,S-033447)毒理性质:
In clinical trials, there was little evidence that baloxavir caused liver injury, either in the form of serum enzyme elevations or clinically apparent liver disease. A proportion of patients with acute influenza A may have minor serum enzyme elevations during the acute illness, but these are independent of therapy and do not appear to be exacerbated by baloxavir.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
巴洛沙韦(1985605-59-1,Baloxavir,Baloxavir acid,S-033447)实验注意事项:
1.实验前需戴好防护眼镜,穿戴防护服和口罩,佩戴手套,避免与皮肤接触。
2.实验过程中如遇到有毒或者刺激性物质及有害物质产生,必要时实验操作需要手套箱内完成以免对实验人员造成伤害
3.实验后产生的废弃物需分类存储,并交于专业生物废气物处理公司处理,以免造成环境污染Experimental considerations:
1. Wear protective glasses, protective clothing and masks, gloves, and avoid contact with the skin during the experiment.
2. The waste generated after the experiment needs to be stored separately, and handed over to a professional biological waste gas treatment company to avoid environmental pollution.
Tags:巴洛沙韦试剂,巴洛沙韦杂质,巴洛沙韦中间体,巴洛沙韦密度,巴洛沙韦溶解度,巴洛沙韦旋光度,巴洛沙韦闪点,巴洛沙韦熔点,巴洛沙韦购买,巴洛沙韦MSDS,
| 产品说明 | 巴洛沙韦(1985605-59-1,Baloxavir,Baloxavir acid,S-033447)可以作为药物杂质对照品以及生物医药类试剂。 |
| Introduction | 巴洛沙韦(1985605-59-1,Baloxavir,Baloxavir acid,S-033447)can be used as a reference substance for drug impurities and reagents,only for research. |
| Application1 | Baloxavir(Baloxavir acid)是甲型和乙型流感病毒聚合酶PA亚基内的一种首创,有效且选择性的帽核苷酸核酸内切酶(CEN)抑制剂。Baloxavir抑制病毒RNA的转录和复制,并具有强大的抗病毒活性。 |
| Application2 | 巴洛沙韦是用于治疗甲型和乙型流感病毒感染的流感帽依赖性核酸内切酶的选择性抑制剂。 |
| Application3 |
1、巴洛沙韦(1985605-59-1,Baloxavir)是流感帽依赖性核酸内切酶的抑制剂,巴洛沙韦可用于治疗A型和B型流感。Baloxavir一次性使用一次,与血清酶升高或临床上明显的肝损伤无关。
2、巴洛沙韦是抗病毒药,巴洛沙韦用于预防或治疗病毒性疾病的药物。它们可能发挥作用的方式包括通过抑制病毒DNA聚合酶来防止病毒复制。与特定的细胞表面受体结合并抑制病毒渗透或脱壳;抑制病毒蛋白质合成;或阻止病毒组装的后期。
3、巴洛沙韦是酶抑制剂 ,巴洛沙韦与酶结合的化合物或试剂,可防止正常的底物-酶结合和催化反应。
4、Baloxavir marboxil是一流的单剂量口服药物,具有新颖的拟议作用机制,已证明对多种流感病毒具有功效,包括对耐oseltamivir耐药株和禽毒株(H7N9,H5N1 )进行非临床研究。Baloxavir是一种聚合酶酸性,依赖于帽的核酸内切酶抑制剂,可用于治疗症状不超过48小时的12岁及以上的急性单纯性流感。它通过结合两个核酸内切酶结合位点之一来阻断病毒增殖,从而抑制了甲型流感病毒和乙型流感病毒株的mRNA合成。Baloxavir可以与含多价阳离子的产物发生相互作用,从而降低其血浆浓度。
| 警示图 | |
| 危险性 | warning |
| 危险性警示 | Not available |
| 安全声明 | H303+H313+H333 |
| 安全防护 | P264+P280+P305+P351+P338+P337+P313 |
| 备注 | 实验过程中防止吸入、食入,做好安全防护 |
| Omoto S, et al. Characterization of influenza virus variants induced by treatment with the endonuclease inhibitor baloxavir marboxil. Sci Rep. 2018 Jun 25;8(1):9633. |
| Noshi T, et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antiviral Res. 2018 Dec;160:109-117. |
| Baloxavir: First Global Approval PMID 29623652; Drugs 2018 Apr; 78(6):693-697 (Review Article) Name matches: baloxavir marboxil baloxavir |
| Marboxil CID 124081896 42 articles Download CSVView in PubMed Baloxavir: First Global Approval PMID 29623652; Drugs 2018 Apr; 78(6):693-697 (Review Article) Name matches: baloxavir marboxil baloxavir |
| Baloxavir: A Novel Antiviral Agent in the Treatment of Influenza PMID 32020174; Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2020 Feb; ?(?): Nam |
1、Modeling mitigation of influenza epidemics by baloxavir
Zhanwei Du, Ciara Nugent, Alison P. Galvani, Robert M. Krug & Lauren Ancel Meyers
Results and discussion Impact of antiviral treatment on the cell-to-cell proliferation of influenza Our within-host model assumes that infected patients have an initial load of drug-sensitive virus that increases via replication and decreases via immune response and antiviral treatment10,11 (Supplementary Fig. 1). We estimated the efficacy with which oseltamivir and baloxavir inhibit viral replication by fitting the model to the results of a recent clinical trial8 that measured the viral loads of 1014 influenza virus-infected patients after treatment with oseltamivir, baloxavir, or a placebo (Table 1). Our model produces viral titer estimates similar to the clinical data, and, like the clinical data, shows that baloxavir inhibits influenza virus replication more effectively than oseltamivir (Fig. 1). Within 1 day of initiating baloxavir or oseltamivir treatment, virus load decreases by an estimated 84% or 56%, respectively, compared with an expected reduction in untreated cases of 39%. The observed differences in the time between symptom onset and the initiation of treatment for patients in the clinical trial accounts for most of the observed variability in virus replication (Fig. 1, standard deviations). We used the fitted model to predict the effectiveness of drug treatment scenarios beyond those tested in the clinical trial, including the initiation of baloxavir or oseltamivir regimens at different times after symptom onset (Supplementary Fig. 3).
2、Baloxavir for the treatment of Influenza in allogeneic hematopoietic stem cell transplant recipients previously treated with oseltamivir
Mirella Salvatore 1, Jennifer M Laplante 2, Rosemary Soave 1, Nina Orfali 1, Markus Plate 1, Koen van Besien 3, Kirsten St George 2
Abstract Background: Seasonal influenza causes significant morbidity and mortality in allogeneic stem cell transplant (SCT) recipients. In this population, influenza virus can replicate for prolonged periods, despite neuraminidase inhibitor treatment, leading to resistance and treatment failure. Baloxavir targets the influenza polymerase and may be an effective treatment option in these patients. Methods: We used baloxavir to treat five allogeneic SCT recipients that were still symptomatic and shedding influenza virus after completing one or more treatment courses of oseltamivir and characterized the viral isolates before and during treatment. Results: Two patients were infected with influenza A/H1pdm09 carrying a neuraminidase variant (H275Y) linked to oseltamivir resistance. Both these two patients were successfully treated with baloxavir. Of the three patients infected with wild-type influenza virus, two cleared the virus after baloxavir treatment, while the third patient developed the polymerase I38T variant linked to baloxavir resistance. Conclusions: Our data suggest that baloxavir treatment can be effective in treating neuraminidase inhibitor-resistant influenza in profoundly immunocompromised patients. Randomized clinical trials are needed to define the role of baloxavir alone and combined with oseltamivir for the treatment of influenza in SCT recipients and other immunocompromised populations. Keywords: antiviral resistance; baloxavir marboxil; influenza A virus; neuraminidase inhibitors; oseltamivir; stem cell transplant.
- 相关产品
-
< >
- 推荐产品
-
< >
- 最新产品
-
< >
新闻
怎么做细胞爬片免疫组化染色实验
细胞爬片免疫组化染色,是通过细胞爬片是让玻片浸在细胞培养基内,细胞在玻片上生长,主要用于组织学,免疫组织化学...
2020/7/20 22:04:33
提取病毒RNA的实验方法
提取病毒RNA方法分别有:异硫氰酸胍的提取病毒RNA方法、TRIzol LS提取法、Trizol法提取法等等...
2020/7/22 20:29:26

9月开学季——助研新学期 范德送好礼
2025/8/28 15:30:55

Waxfilm 实验室封口膜:技术与国际市场的双重突破
在实验室耗材领域,封口膜是保障实验准确性与稳定性的关键产品之一。近年来,Waxfilm?实验室封口膜凭借其卓...
2025/5/13 13:03:40

Waxfilm实验室封口膜的5大突破
Waxfilm实验室封口膜作为生物功能膜领域的国产技术突破和品牌突破,是生物领域中国技术发展的缩影。
2025/5/6 17:02:07
各种微流控芯片键合方法的优缺点
微流控芯片键合:目前主要有激光焊接、热压键合、胶键合、超音波焊接,每种方法都有各自的优缺点。本文主要介绍聚酯...
2023/7/28 10:43:09

新一代微流控键合解决方案
微流控键合解决方案:微流控芯片制造的一个重要环节,也是最容易被忽视的--芯片键合。其中一个重要因素是:微流控...
2023/7/27 12:44:28
荧光素钾盐使用说明
D-荧光素钾盐(K+)设计用于体外和体内生物发光测定。D-荧光素的质量和纯度对于获得良好和可重复的结果至关重...
2023/7/20 11:05:11
如何选BSA(牛血清白蛋白)
如何选BSA(牛血清白蛋白):牛血清白蛋白(BSA)有多种形式,如何选择适合自己的牛血清白蛋白(BSA)是一...
2023/2/14 13:09:18

牛血清白蛋白(BSA)常见问题
牛血清白蛋白(BSA)常见问题:牛血清白蛋白(BSA)在实验室中是通用的,可用于蛋白质印迹、细胞组织培养、P...
2022/10/19 9:39:51




 购物车
购物车 



